EC Number   |
Reference   |
|---|
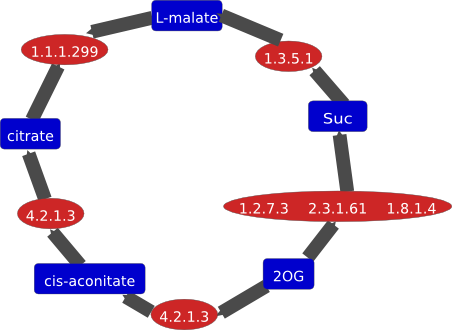    5.4.2.8 5.4.2.8 | 12-15 mg/ml PMM/PGM solution in 10 mM MOPS, crystals grow by hanging-drop vapor diffusion from 1.4 M sodium/potassium tartrate and 100 mM Na-HEPES, pH 7.5 from drops containing 0.002 ml protein and 0.002 ml of well buffer, crystals diffract to 1.75 A |
649138 |
    5.4.2.8 5.4.2.8 | crystal structure of the selenomethionine-substituted PMM/PGM at 2.2 A resolution, crystal structure of S108A mutant at 1.75 A resolution |
653900 |
    5.4.2.8 5.4.2.8 | free enzyme and in complex with glucose 1,6-bisphosphate at 2.1 and 2.9 A resolution, resp. Comparison with structure of human enzyme |
681400 |
    5.4.2.8 5.4.2.8 | in complex with inhibitor xylose 1-phosphate or slow substrate ribose 1-phosphate. Both ligands induce an interdomain rearrangement, using different enzyme-ligand interactions |
677397 |
    5.4.2.8 5.4.2.8 | isoform alpha-PMM1 in the open conformation, with and without bound substrate alpha-D-mannose 1-phosphate. Protein consists of two domains, the cap and the core. Substrate phosphate group is at a positively charged site of the cap domain, suggesting that substrate binds first to the cap and then is swept into the active site, thereby facilitating its closure over the core domain |
680647 |
    5.4.2.8 5.4.2.8 | mutant enzyme P368G, hanging drop vapour diffusion method |
690999 |
    5.4.2.8 5.4.2.8 | phospho- and dephospho-enzyme in complex with reaction intermediate glucose 1,6-bisphosphate at 1.9 and 2.0 A |
680649 |
    5.4.2.8 5.4.2.8 | purified recombinant detagged enzyme, hanging drop vapor diffusion and microseeding techniques, 1.3 to 1.4 M sodium/potassium tartrate and 100 mM HEPES, pH 7.5, X-ray diffraction structure determination and analysis at 1.8 A resolution, modeling |
727988 |
    5.4.2.8 5.4.2.8 | purified recombinant detagged enzyme, hanging drop vapour diffusion, from 2 M (NH4)2SO4, 0.2 M NaCl, 0.1 M sodium cacodylate, pH 6.0, 20°C, X-ray diffraction structure determination and analysis at 1.86 A resolution, molecular replacement using a modified form of the Leishmania mexicana enzyme, PDB ID 2i54 |
728329 |





