EC Number   |
Reference   |
|---|
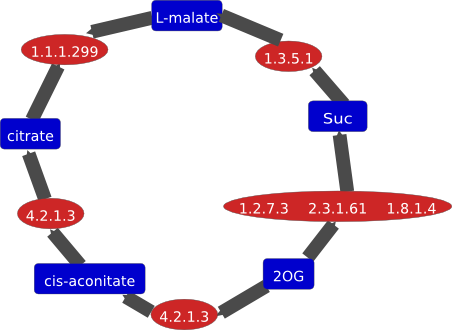    1.1.1.35 1.1.1.35 | 50 mM N(2-acetamido)-2-iminodiacetic acid, pH 6.5, polyethylene glycol 4000, 5 mM NAD+ hanging drop, crystals within 3 to 5 days at 18°C, enzyme structure is compromised of two domains, a NAD+-binding domain and a helical C-terminal domain |
286588 |
    1.1.1.35 1.1.1.35 | 50% saturation with ammonium sulfate solution, 0.1 M potassium phosphate, pH 6.8, 1 mM EDTA, 2 mM beta-mercaptoethanol, 4°C, crystals appear after 2 days |
286606 |
    1.1.1.35 1.1.1.35 | apoenzyme, hanging drop vapor diffusion method, using 1.25% (w/v) PEG 400, 2.2 M ammonium sulfate, 0.1 M HEPES-NaOH pH 7.5. Enzyme in complex with NAD+, hanging drop vapor diffusion method, using 17.5% (w/v) PEG 3350, 200 mM sodium thiocyanate |
760296 |
    1.1.1.35 1.1.1.35 | dialysis against 40% saturated ammonium sulfate containing 100 mM phosphate, 2 mM beta-mercaptoethanol, 1 mM EDTA, pH 6.9, 7.5 or 8.2, vapor diffusion crystallization, crystals are obtained in the ammonium sulfate saturation range of 41% to 48% |
286608 |
    1.1.1.35 1.1.1.35 | hanging drop vapour diffusion method, mixing of 30 mg/ml protein in 40 mM Tris-HCl, pH 8.0, 1 mM DTT, with reservoir solution containing 2 M ammonium sulfate, 0.1 M CAPS, pH 10.5, and 0.2 M lithium sulfate, 22°C, 7 days, X-ray diffraction structure determination and analysis at 2.3 A resolution, molecular replacement method and structure modeling |
739812 |
    1.1.1.35 1.1.1.35 | polyethylene glycol, pH 8, orthorhombic crystals, 2.7 A resolution, crystallisation at pH 5 leads to trigonal space group |
286594, 286596 |
    1.1.1.35 1.1.1.35 | purified recombinant detagged enzyme, hanging drop vapour diffusion method, 10 mg/ml protein in 25 mM sodium acetate trihydrate, pH 5.0, and 300 mM sodium chloride is mixed with an equal volume of reservoir solution, containing 21% PEG 3350, 0.2 M sodium chloride, 0.1 M MES, pH 6.5, for parallelepiped-shaped crystals and 23% PEG 3350, 0.2 M sodium chloride, 0.1 M bicine, pH 8.0, for cuboid-shaped crystals, equilibration against 0.20 ml reservoir solution, 3-5days at 18°C, X-ray diffraction structure determination and analysis of parallelepiped-shaped cuboid-shaped crystal at 1.60 A and 2.20 A resolution, respectively |
739806 |
    1.1.1.35 1.1.1.35 | purified recombinant detagged wild-type enzyme, crystals are grown by hanging drop method from 23% PEG 3350, 0.2 M sodium chloride, 0.1M N,N-bis (2-hydroxyethyl)glycine, pH 8.0, X-ray diffraction structure determination and analysis at 2.20 A resolution, molecular replacement with the human HAD structure as search model, PDB ID 3had |
741307 |
    1.1.1.35 1.1.1.35 | purified recombinant enzyme in apoform, as selenomethionine-labeled enzyme, and in complex with substrates acetoacetyl-CoA and NAD+, hanging drop vapour diffusion method, mixing of 50 mg/ml wild-type protein or selenomethionine-labeled enzyme in 40 mM Tris-HCl, pH 8.0, 1 mM DTT, with reservoir solution containing 2 M ammonium sulfate, 0.1 M sodium cacodylate, pH 6.5, and 0.2 M sodium chloride, 22°C, 7 days, X-ray diffraction structure determination and analysis at 2.42-2.7 A resolution, molecular replacement using the crystal structure of the apo-form of RePaaH1, and structure modeling |
739989 |
    1.1.1.35 1.1.1.35 | purified recombinant His6-tagged wild-type enzyme in apoform and in complex with substrates acetoacetyl-CoA and NAD+, hanging drop vapour diffusion method, mixing of 30 mg/ml protein in 40 mM Tris-HCl, pH 8.0, 1 mM DTT, with or without 20 mM NAD+, and 20 mM acetoacetyl-CoA, with reservoir solution containing 0.2 M Li2SO4, 0.1 M CAPS, pH 10.5, and 2 M ammonium sulfate, 22°C, 7 days, X-ray diffraction structure determination and analysis at 1.8-2.54 A resolution, molecular replacement and structure modeling |
740854 |





